Why does repeated handwashing damage your skin?
Why do we sometimes have the impression that hand sanitizer burns the skin?
Why does our skin feel a little tighter after every use?
Why does its tolerance for this essential hygiene fall?
The negative effects of soap and hand sanitizer gel on the skin are linked to their disinfectant properties. Their positive impact against viruses is also their negative impact on our skin.
Why are hand sanitizers effective at stopping viruses? The virucide power of antibacterial hand lotions lies in their capacity to destroy the lipidic membranes that encase viruses.
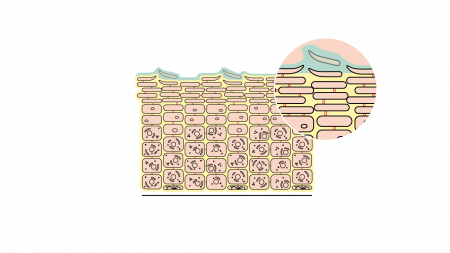
The problem? Our skin also contains large amounts of lipids. Intensive use of hydroalcoholic gels and alcohol-based hand sanitizers therefore damages the lipids that form a protective barrier on the skin’s surface, making it more fragile. Without lipids (fats), the skin becomes very dry and more susceptible to the unpleasant conditions previously listed.
Undermining the skin’s barrier function
Our skin is designed to envelop and protect us from external irritations. But if an external factor destabilises its natural balance, the skin can no longer carry out this protective role correctly. This is exactly what alcohol-based hand sanitizers do. By protecting us from contamination, they damage the skin’s integrity and destroy precious lipids.